|
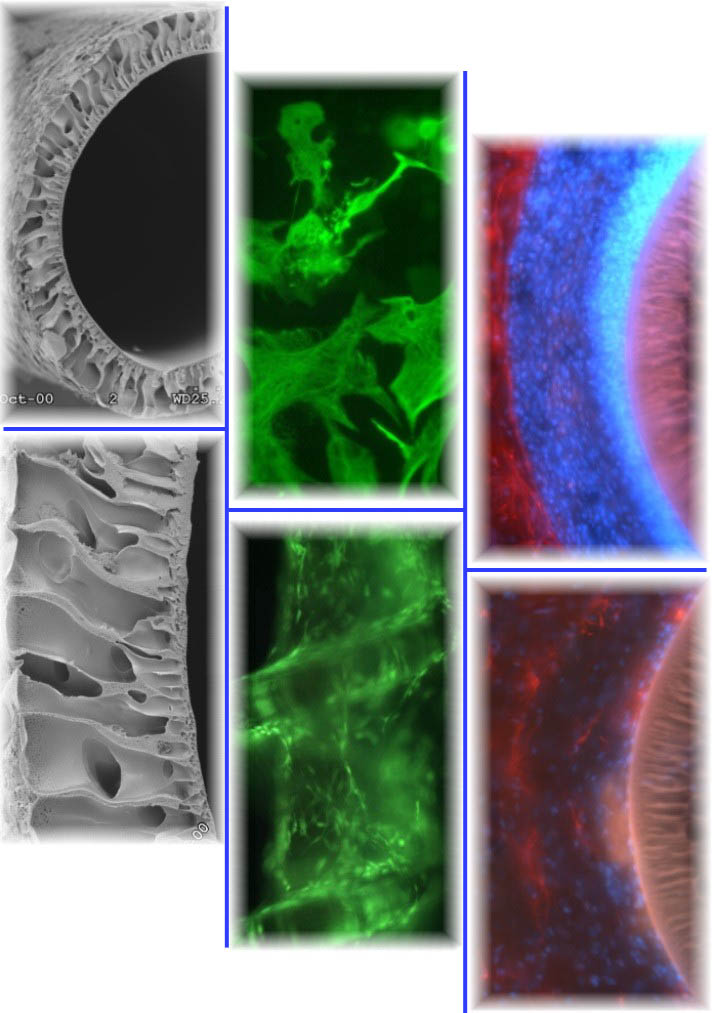 Insertion of foreign materials and the penetrating injury required to
implant such materials into the CNS results in a wound healing response mounted by blood and brain-derived reactive cells. The ultimate impact of a material on this response is often referred to as its
biocompatibility. Generally, the concept of an acceptable or good response has been loosely and often subjectively applied to any material that elicits a minimal response in tissue adjacent to the implant
interface. What can be reliably gleaned from the biocompatibility literature is that regardless of their composition and intended applications, most indwelling materials in the CNS elicit one of several
characteristically similar chronic host wound healing reactions. The chronic result is the formation of a glial-fibroblastic scar, that is
characteristically inhibitory to axon growth, is believed to function as a diffusive barrier, and is thought to reduce the performance of chronic recording/stimulating devices by insulating the device from the
surrounding brain tissue. Insertion of foreign materials and the penetrating injury required to
implant such materials into the CNS results in a wound healing response mounted by blood and brain-derived reactive cells. The ultimate impact of a material on this response is often referred to as its
biocompatibility. Generally, the concept of an acceptable or good response has been loosely and often subjectively applied to any material that elicits a minimal response in tissue adjacent to the implant
interface. What can be reliably gleaned from the biocompatibility literature is that regardless of their composition and intended applications, most indwelling materials in the CNS elicit one of several
characteristically similar chronic host wound healing reactions. The chronic result is the formation of a glial-fibroblastic scar, that is
characteristically inhibitory to axon growth, is believed to function as a diffusive barrier, and is thought to reduce the performance of chronic recording/stimulating devices by insulating the device from the
surrounding brain tissue.
The inflammatory and subsequent scarring response to indwelling materials in the CNS is perhaps the single
largest hurdle to achieving success using biomaterial-based therapeutics. As a result, we are focused on the
discovery and application of reagents that may be used to control the host response at biomaterial interfaces.
Our research efforts are currently focused on biologically active compounds produced by cells that function to modulate tissue remodeling within the CNS.
|

